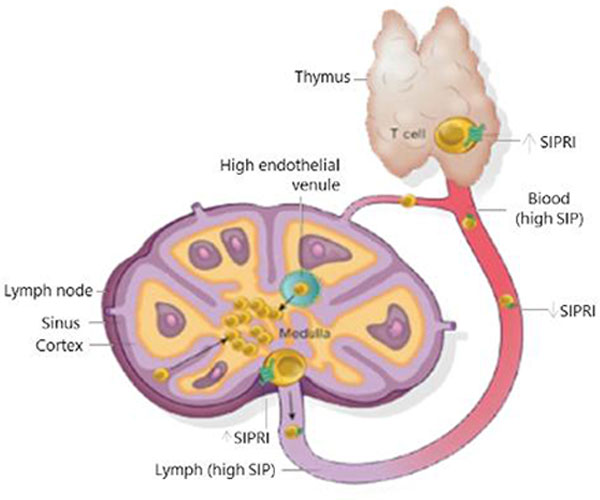
Vibozilimod is a highly selective, second-generation S1P1 receptor-1 (S1PR1) agonist. Internalization of S1PR1 by Vibozilimod prevents the egress of lymphocytes from the lymph nodes to the peripheral circulation. This immunomodulatory action of Vibozilimod has a potential to be developed as a safer alternative to the Janus Kinase inhibitors for treatment of various inflammatory and autoimmune disorders.
Fingolimod is an approved first-generation S1PR1 agonist indicated for treatment of multiple sclerosis. However, use of fingolimod is associated with side effects attributed to its non-selective action on other S1P receptors, like the S1PR3 receptors. Vibozilimod is a highly selective second-generation S1PR1 agonist with 10,000-fold selectivity for S1PR1 over S1PR3. Multiple selective second-generation S1PR1 modulators are approved (siponimod, ponesimod and ozanimod), but only for non-dermatology indications. The S1PR1 agonist class of compounds are associated with transient cardiac conduction abnormalities.
Vibozilimod is being developed for treatment of dermatological conditions. Extensive assessment of single and multiple ascending doses of vibozilimod was undertaken in the Phase I clinical program. Vibozilimod has been evaluated up to a dose of 6 mg daily in the multiple dose studies in healthy volunteers. A dose titration scheme successfully prevented any clinically relevant effect of Vibozilimod on cardiac conduction at the anticipated therapeutic dose.
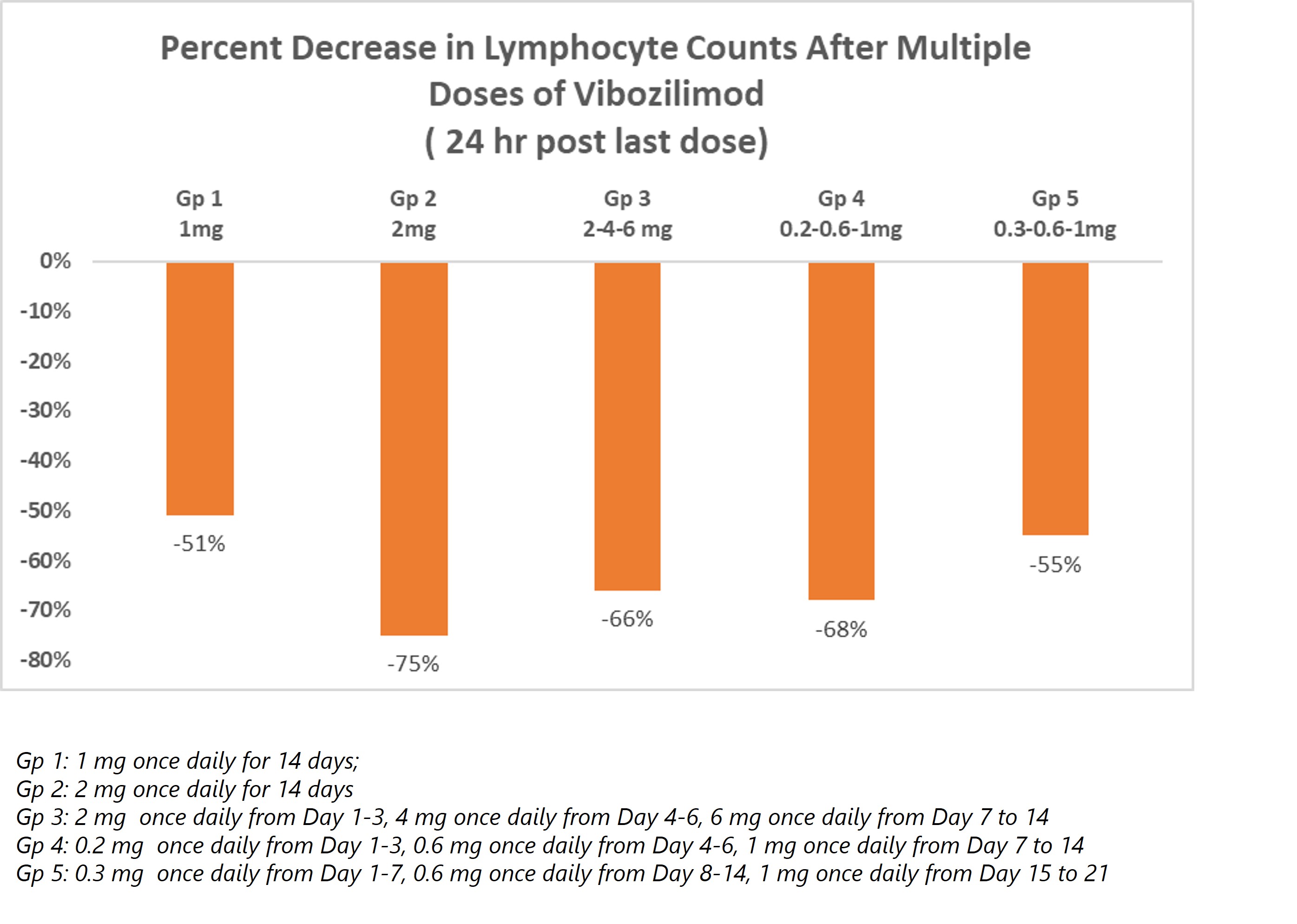
Vibozilimod demonstrated clinically relevant reduction in lymphocyte counts in the Phase I program. This pharmacodynamic effect is believed to be predictive of efficacy in multiple dermatological indications thereby prompting advancement of the drug to the next stage of clinical development.
Two phase 2b dose-ranging clinical studies of Vibozilimod are ongoing; one study in moderate to severe plaque psoriasis and another in atopic dermatitis. Efficacy and safety readouts from these studies are expected in Q1 of 2025, post which a Phase 3 program will be initiated post consultation with the relevant regulatory agencies.